Clin Neurophysiol. Author manuscript; available in PMC 2018 Jun 4.
Published in final edited form as:
Clin Neurophysiol. 2017 Sep; 128(9): 1774–1809.
Published online 2017 Jun 19. doi: 10.1016/j.clinph.2017.06.001
PMCID: PMC5985830
NIHMSID: NIHMS968774
PMID: 28709880
Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines
A. Antal,a,* I. Alekseichuk,a M. Bikson,b J. Brockmöller,c A.R. Brunoni,d R. Chen,e L.G. Cohen,f G. Dowthwaite,g J. Ellrich,h,i,j A. Flöel,k F. Fregni,l M.S. George,m R. Hamilton,n J. Haueisen,o C.S. Herrmann,p F.C. Hummel,q,r J.P. Lefaucheur,s D. Liebetanz,a C.K. Loo,t C.D. McCaig,u C. Miniussi,v,w P.C. Miranda,x V. Moliadze,y M.A. Nitsche,z,aa R. Nowak,ab F. Padberg,ac A. Pascual-Leone,ad W. Poppendieck,ae A. Priori,af S. Rossi,ag P.M. Rossini,ah J. Rothwell,ai M.A. Rueger,aj G. Ruffini,ab K. Schellhorn,ak H.R. Siebner,al,am Y. Ugawa,an,ao A. Wexler,ap U. Ziemann,aq M. Hallett,ar,1 and W. Paulusa,1
Author information Copyright and License information PMC Disclaimer
The publisher's final edited version of this article is available at Clin Neurophysiol
Abstract
Low intensity transcranial electrical stimulation (TES) in humans, encompassing transcranial direct current (tDCS), transcutaneous spinal Direct Current Stimulation (tsDCS), transcranial alternating current (tACS), and transcranial random noise (tRNS) stimulation or their combinations, appears to be safe. No serious adverse events (SAEs) have been reported so far in over 18,000 sessions administered to healthy subjects, neurological and psychiatric patients, as summarized here. Moderate adverse events (AEs), as defined by the necessity to intervene, are rare, and include skin burns with tDCS due to suboptimal electrode-skin contact. Very rarely mania or hypomania was induced in patients with depression (11 documented cases), yet a causal relationship is difficult to prove because of the low incidence rate and limited numbers of subjects in controlled trials. Mild AEs (MAEs) include headache and fatigue following stimulation as well as prickling and burning sensations occurring during tDCS at peak-to-baseline intensities of 1–2 mA and during tACS at higher peak-to-peak intensities above 2 mA.
The prevalence of published AEs is different in studies specifically assessing AEs vs. those not assessing them, being higher in the former. AEs are frequently reported by individuals receiving placebo stimulation. The profile of AEs in terms of frequency, magnitude and type is comparable in healthy and clinical populations, and this is also the case for more vulnerable populations, such as children, elderly persons, or pregnant women. Combined interventions (e.g., co-application of drugs, electrophysiological measurements, neuroimaging) were not associated with further safety issues.
Safety is established for low-intensity ‘conventional’ TES defined as <4 mA, up to 60 min duration per day. Animal studies and modeling evidence indicate that brain injury could occur at predicted current densities in the brain of 6.3–13 A/m2 that are over an order of magnitude above those produced by tDCS in humans. Using AC stimulation fewer AEs were reported compared to DC. In specific paradigms with amplitudes of up to 10 mA, frequencies in the kHz range appear to be safe.
In this paper we provide structured interviews and recommend their use in future controlled studies, in particular when trying to extend the parameters applied. We also discuss recent regulatory issues, reporting practices and ethical issues. These recommendations achieved consensus in a meeting, which took place in Göttingen, Germany, on September 6–7, 2016 and were refined thereafter by email correspondence.
Keywords: tDCS, tACS, TES, Safety, Adverse events
1. Introduction
The aim of this review is to update the safety of low-intensity electric stimulation based on available published research and clinical data in animal models and in human studies until the end of 2016. The essentials of the present manuscript were agreed upon at a two-day safety conference held in Göttingen, Germany on 6–7th September, 2016. Participants included research and clinical experts from neurophysiology, neurology, cognitive neuroscience and psychiatry. Representatives of transcranial electrical stimulation (TES) equipment manufacturers contributed to regulatory issues.
For the purposes of this review, data from published articles that encompassed more than 18,000 stimulation sessions in ~8000 subjects, according to a recent review (Bikson et al., 2016), using low intensity stimulation (<4 mA; see definitions below) up to 60 min duration/day were included. Literature searches investigated by experts on the related fields covered studies using transcranial direct current stimulation (tDCS), alternating current stimulation (tACS) and random noise stimulation (tRNS), with key words Adverse Events (AE) or Reactions (AR) and/or safety (see definitions below), in order to assess stimulation-related risks and to better understand of the risk-benefit ratio of these procedures. We relied on summarizing and interpreting data on (1) available animal studies, (2) computational modeling and (3) testing in human trials, including reports on healthy subjects, patients and on theoretically vulnerable populations, such as children, elderly and pregnant women. With regard to animal data the main effort was devoted to understanding the translation of findings to human applications (e.g., the relationship of dose of the stimulation and safety). Concerning patients, only the most frequently investigated clinical groups were included (major depression, chronic pain and stroke), because of lack of data in other populations. Special stimulation conditions that are increasingly used during the last years, e.g., combination of TES with other methods, such as stimulating patients with intracranial implants, combination of TES with transcranial magnetic stimulation (TMS) or functional magnetic resonance imaging (fMRI), as well as “do it yourself” use of TES for neuro-enhancement purposes, were also considered, because of the theoretical increased risk in these conditions. Furthermore, other stimulation settings than ‘transcranial’, in which recent safety data are available, were also integrated (e.g., using transcutaneous spinal direct current stimulation (tsDCS) and applying optic nerve stimulation (ONS)).
In general, human studies that evaluate parameters of neuronal damage, such as neuron specific enolase (NSE), magnetic resonance imaging (MRI) (Nitsche et al., 2004), electroencephalography (EEG), and neuropsychological tests (Iyer et al., 2005; Tadini et al., 2011) support the safety of tDCS. However, it is also important to underscore the fact that the safety of low intensity TES is mostly derived from an analysis of secondary outcomes in TES clinical trials assessing efficacy as the primary outcome.
In this paper, we first provide an overview of the technical parameters and basic principles of low intensity TES used alone or combined with other methods, safety aspects of the stimulation with a summary of the published AEs in healthy subjects and different patient populations. The presumed mechanisms of TES and the efficacy of TES in eliciting desired outcomes are not relevant for the scope of this review except for instances, in which they inform about safety. Other stimulation methods that are applying specific (brand) waveforms or conditions, such as cranial electrical stimulation (CES) are also not incorporated here, but have been comprehensively reviewed by other authors (Mindes et al., 2015). We also present recent regulatory issues and recommend rules for reporting in research and clinical practice, and finally we summarize existing data and provide recommendations for future safety monitoring. Consensus with regard to the definitions, recommendations, etc. were reached by using a modified Delphi method, in this case a structured interactive communication technique (Kleymeyer, 1976). The experts first summarized safety data related to their fields and answered questions in more rounds. The key results were presented and discussed in Göttingen at the meeting. After that the experts were encouraged to support or revise their earlier answers in light of the replies of other members of the panel and in response to reviewers’ critiques.
1.1. Basic aspects: nomenclature and explanations
We adopt suggested definitions as already published (e.g., Bikson et al., 2016; Woods et al., 2016) except that we chose the term “burden” instead of “tolerability” in accordance with the Declaration of Helsinki (1964) (Last revision 2013). The following terms are used in this paper:
Low intensity TES: This is defined as intensities <4 mA, a total stimulation duration of up to 60 min per day, and using electrode sizes between 1 cm2 and 100 cm2 (delivering ≤7.2 coulombs of charge) (Bikson et al., 2016) to apply frequencies between 0 and 10,000 Hz. The intensity of tDCS is always defined as peak-to-baseline, while with tACS peak-to-baseline or peak-to-peak intensities can be used. The type of current is direct current or bipolar alternating current (Guleyupoglu et al., 2013).
Safety can probably only be considered in relative terms. According to the definition of the European Medical Device Directive, ‘safe’ is a condition where all risks are accepted risks (Annex I; § I. General Requirements). However, all stimulation protocols carry a certain degree of risk and could cause problems in specific circumstances. Many problems cannot be detected until extensive research or clinical experience is gained. The current approach in this field is to estimate the potential of a protocol becoming a hazard that could result in safety problems (e.g., using too high intensities or too long durations of stimulation). Hazard is a potential for an AE. Risk is a measure of the combination of the hazard, the likelihood of occurrence of the AE and the severity (Altenstetter, 2003; McAllister and Jeswiet, 2003) (See also: http://www.who.int/medical_devices/publications/en/MD_Regulations.pdf). The conclusion that a procedure is safe is based on a comprehensive and unbiased documentation of all AEs in relation to the frequency of application of the procedure. Risk must be differentiated from burden, a procedure may be burdensome (e.g., produce much discomfort) but nevertheless safe (e.g., not having any relevant risk for permanent damage).
Generally and according to the Common Terminology Criteria for Adverse Events (AEs) (https://evs.nci.nih.gov/ftp1/CTCAE/Archive/CTCAE_4.02_2009-09-15_QuickReference_5x7_Locked.pdf), AEs are undesirable, uncomfortable or harmful effects that are observed after a medical intervention that may or may not be causally related to it. Here, we prefer the term AE to the term Side Effect (SE), which is frequently employed synonymously to describe AEs. A SE should be a consequence different than the intended effect, and might be good or bad (beneficial or adverse). An example of a good SE might be an improvement of memory by an intervention for depression. An AE is by definition always bad. In the context of the present paper the term SE will not be used in accordance with recommendations in the ICH guidelines (Baber, 1994; Food and Drug Administration, 2011). According to this classification, a mild AE (MAEs – grade 1) is defined as involving mild symptoms for which no medical treatment is necessary (i.e. skin redness or tingling during tDCS), while a moderate AE (grade 2) indicates the need of local or noninvasive treatment (e.g., in the case of TES, the local application of a cream after a skin burn). Serious AEs (grade 3) (SAE) are severe or medically significant but not immediately life-threatening events, include the requirement for inpatient hospitalization or prolongation of hospitalization. Life threatening SAEs include any event that may be life threatening (grade 4) or death from the AE (grade 5).
Suspected Adverse Reaction (AR) means any AE for which there is a reasonable possibility (causality is probable, likely or certain) that the intervention caused the AE (Baber, 1994; Food and Drug Administration, 2011). The distinction between AE and AR is not always clear, first because causality often cannot be proven unambiguously, and second because some effects (e.g., sedation) may be in some instances good but in other instances bad for the patient. Another point to be considered is unexpectedness. An AE or suspected AE is generally considered unexpected if it is not listed in the information brochure or is not listed at the specificity or severity level that has been observed or it is not consistent with the risk information described in the investigational plan (FDA regulations, 21CFR312.32, safety reporting). Unexpected ARs require particular attention because their correlation with the procedure may be neglected. If for example, someone is treated using tDCS and is hit by a car an hour later, this is usually not considered as AR. However, if it is due to sedation and cognitive impairment it may indeed be an AR. Corresponding to the definitions above, mild, moderate and severe ARs may be defined. The risk-benefit ratio is the overall ratio of all potential benefits of a procedure divided by all the ARs of a procedure. Usually, a procedure is only acceptable if the beneficial effects outweigh the risks.
2. Assumptions regarding dose-response relationship, animal studies
TES dose is defined by all of the parameters of the stimulation device that affect the generated electric field (EF) in the body with units of V/m (or, equivalently, mV/mm) (Peterchev et al., 2012). This includes the parameters of the electrode montage (skin contact area), the waveform applied to the electrodes and at the case of tACS, the stimulation frequency.
The parameters delivered by the stimulation equipment are well defined and reproducible, while other influencing factors are not (e.g., individual tissue properties and anatomy, age, gender, baseline neurotransmitter concentrations, genetics, dynamic state of the brain before and during stimulation) or only barely controllable. Nevertheless, they shape the physiological responses to the stimulation and should therefore be considered along with the dose selection. Due to the high individual variability of these factors the electrical stimulation dose cannot fully determine the magnitude of the physiological or therapeutic outcome since it cannot be guaranteed that given the same doses the outcomes of stimulation will be the same. Furthermore, the indirect effects of TES, e.g., afferent low threshold stimulation of peripheral nerves, cranial nerves and retina cannot be avoided and can lead to neuro-modulatory effects of their own or in conjunction with brain stimulation. This presents a challenge to researchers and clinicians when finding the ‘optimal’ dose for a given application. Unfortunately, due to these uncontrollable factors and additional putative mechanisms that are initiated during stimulation (activation of glial cells, vasodilation, changes in blood-barrier permeability, etc.), the current state of knowledge of the physiological mechanisms of TES remains limited. At present, in most studies the dose is chosen based on previously published data, prior clinical experience, individual measures such as thresholds, computational models, summary metrics (including all parameters: intensity, electrode size, stimulation duration) and safety considerations based on human and animal experimental data.
In vivo, the dosage induced by tDCS may, in a first approximation, be the EF as described by the charge density, given as (current [A] * stimulation duration [s])/electrode contact size [m2]). However, the relation of this to EF or time integrated EF on the cortex is not simple and certainly not linear (Miranda et al., 2009; Ruffini et al., 2013a). In humans, tDCS with approximately 1 mA using standard contact electrodes (sizes between 16 and 35 cm2) results in charge densities ranging from 170 to 480 C/m2 (Liebetanz et al., 2009). In animal experiments, much higher charge densities, sometimes exceeding the doses in human low intensity TES studies by several orders of magnitude, have been applied. In an animal study, safety limits were determined histologically by applying DC of increasing intensities directly to the rat cortex using an epicranial wet electrode (Liebetanz et al., 2009). At current densities between 14.3 and 28.7 mA/cm2, corresponding to a charge density threshold below 52,400 C/m2, no histologically detectable brain lesions were induced. In a histology-based (hematoxylin & eosin staining) study, safety limits were determined by applying increasingly powerful tDCS regimes through an open epicranial wet electrode (Liebetanz et al., 2009). Combined with updated safety data in rats, this threshold approximation obtained from the rat experiments was estimated to be over one order of magnitude higher compared to current clinical protocols (Bikson et al., 2016). But many uncertainties in the translation of animal studies to human experiments remain.
3. Interaction of EF with tissue, electroporation, galvanotaxis
A variety of montages ranging from two large, pad electrodes to arrays of smaller electrodes are used for tDCS (Alam et al., 2016) with a typical current of 1–2 mA (0.03–2 mA/cm2 current to electrode area ratios depending on the electrode size); this results in cortical EF strengths of up to 0.4–0.8 V/m (Ruffini et al., 2013b) with typical durations of 10–30 min. Both the applied current and the resulting brain EFs are ~1000-fold lower than those for pulsed stimulation used for electroconvulsive therapy (ECT) (Alam et al., 2016). These small EFs are considered to be below the intensity required to evoke action potentials in a resting cell (Radman et al., 2009), but likely modify spontaneous firing rates and ongoing processes such as plasticity that are sensitive to polarization levels (Fritsch et al., 2010; Jackson et al., 2016; Ranieri et al., 2012), and over time may induce molecular or structural changes. Indeed, neuronal network activity generates its own endogenous EFs in brain extracellular spaces and these, in turn, influence network firing (Frohlich and McCormick, 2010). The measured field strength in the ferret visual cortex was around 2–4 V/m and altered the neuronal transmembrane potential (Vmem) by 0.5 and 1.3 mV, respectively (Frohlich and McCormick, 2010).
Many developing and regenerating tissues generate steady electrical gradients, and many cell types respond to these signals with directed migration, enhanced migration rates and regulated proliferation and differentiation. This migration is termed galvanotaxis and occurs at physiological field strengths of 5–150 mV/mm. With very long stimulation duration, galvanotaxis may play a role in the safety of tDCS. The mechanisms that drive cell migration in an EF include induced asymmetries of electrically charged membrane proteins and local activation of downstream signaling pathways, e.g., the neuronal nicotinic ACh receptor in nerve growth cones coupled to cAMP signaling, and the EGF receptor at the leading edge of corneal epithelial cells coupled to ERK1/2 and PI3K signaling (McCaig et al., 2005). Recent additions to this array of molecular players include ATP and the P2Y1 receptor, which transduce the EF into cathodal neuronal migration. The concept involves EF-induced neuronal ATP release and autocrine feedback on its own asymmetrically distributed receptors (Cao et al., 2015), a concept first raised for ACh in neuronal growth cones (Erskine and McCaig, 1995).
The brain microenvironment modulates migration. Keratinocyte fragments migrate anodally and intact parent cells cathodally. Anodal migration is myosin II dependent, whilst the PI3kinase pathway underpins cathodal cell migration (Sun et al., 2013). In cytoskeletal terms, the Arp2/3 complex is required for oligodendrocyte precursors to migrate cathodally (Li et al., 2015). Glioblastoma cells migrate anodally in 2D culture, but switch to cathodal migration in 3D hyaluronic acid plus collagen cultures. Myosin II is not needed for the 2D anodal migration, but is required for 3D cathodal migration. By contrast, PI3kinase regulates the 2D anodal response (Huang et al., 2016).
Hypoxia enhances galvanotaxis of mouse keratinocytes, which is important in wound healing (Guo et al., 2015). Hypoxia is likely not to be present in the healthy brain but it may play a specific role in acute stroke. DC stimulation markedly increases tissue oxygen consumption (Pulgar, 2015), so galvanotaxis could theoretically be enhanced, or its threshold reduced in brain regions that are excessively stimulated by tDCS. Besides this, hypoxia can stimulate stem cell differentiation, and tDCS of regions containing neural or cancer stem cells, such as glioblastomas, may raise specific problems (Bath et al., 2013; Guo et al., 2015). However, at the present stage it is unclear if this needs specific considerations in terms of safety aspects, since longer stimulation durations and intensities higher than those applicable in human tDCS usually have be used for the effects reported in animal studies.
Finally, several studies found that glia cells are involved in the mechanisms underlying tDCS (Gellner et al., 2016; Monai et al., 2016; Ruohonen and Karhu, 2012). Rat cortical astrocytes migrate anodally and show increased proliferation in an EF of 40 mV/mm (Baer et al., 2015). Nerves and Schwann cells have a galvanotaxis threshold of ca. 5 mV/mm (McCaig et al., 2005), which is close to the field generated by tDCS (~1 mV/mm). However, tDCS does not induce directed migration of labeled neural stem cells transplanted into the rat brain (Keuters et al., 2015).
At much higher EF strengths, pulses of DC stimulation have been used for electroporation to create nanopores in the plasma membrane to deliver chemotherapeutic drugs or gene therapies intracellularly, to sterilize foodstuffs and to ablate tumor tissue. Irreversible electroporation (IRE) uses DC short pulses of high voltage 3000 V and 50 A current delivered to a target volume of around 50–70 mm3. This gives rise to EFs of around 8000 V/m, which are about 1000 times stronger than the endogenous, steady DC EFs that drive galvanotaxis. IRE uses msec pulses, is minimally invasive and carried out under visual control using CT or MRI imaging. Tumor ablation requires ~100 pulses and these are delivered between heartbeats to avoid arrhythmias. This non-thermal technique has also been used to ablate tumors in pancreas, lung, kidney, gastrointestinal tract, brain, breast, cervix, prostate and sarcomas (Lu et al., 2013; Paiella et al., 2015; Ting et al., 2016).
Conclusions and recommendations: Although with very long stimulation and much higher intensity than in currently applied approaches galvanotaxis may possibly play a role in tDCS, there is yet no conclusive in vivo evidence in either animal models or humans whether any cells close to the stimulation site have migrated away from or toward the electrodes, therefore, more research is needed in this field. While studies on electroporation have shown additive effects of pulsed DC electrical fields, the intensities needed for electroporation remain orders of magnitude above tDCS. Furthermore, the relative sensitivity of cell types (neurons, astrocytes, endothelial cells, etc.) have not been well studied either.
3.1. TES and tissue inflammation
Inflammation in the central nervous system (CNS), i.e., neuroinflammation, is mediated by both brain-resident microglia and invading blood-borne immune cells. Neuroinflammation plays a pathophysiological role not only in classic neuroimmunological diseases, but also in various other neurological disorders such as stroke (Le Thuc et al., 2015) and traumatic brain injury (Loane and Kumar, 2016), as well as in neurodegenerative diseases such as Parkinson’s disease (PD) (Tansey and Goldberg, 2010) and Alz-heimer’s disease (AD) (Heneka et al., 2015).
DC fields affect the alignment and migration of various cultured immune cells (Pelletier and Cicchetti, 2014). Resting murine BV2 microglia cells change their morphology in the EF at 100 V/m and adopt an activated phenotype (Pelletier et al., 2014). Of note, activated BV2 microglia cells do not respond to high-voltage EFs (50–100 V/m) in the same way as resting microglia, but rather react with a decrease in their viability (Pelletier et al., 2014).
Anodal tDCS with 4 kC/m2, a charge density about 10 times higher than a regular human dose, down-regulates inflammatory mediators in the hippocampus of rats subjected to chronic, stress-induced pain (Spezia Adachi et al., 2012). Likewise, anodal tDCS with a charge density of 99 kC/m2 – about 200 times higher than a regular human dose – decreases the number of activated microglia in the healthy mouse brain (Pikhovych et al., 2016). In contrast, electric stimulation with an even higher charge densities may up-regulate inflammatory processes (Rueger et al., 2012), suggesting that higher charge densities may induce subtle tissue damage and trigger an inflammatory response.
TES could enhance functional recovery after stroke and considering the potentially beneficial effects in the sub-acute phase after cerebral ischemia (Floel and Cohen, 2010), this could be consistent with the time line of post-ischemic neuroinflammatory processes (Dirnagl et al., 1999). Cathodal tDCS at ~66 kC/m2 – around 200 times higher than a regular human dose – applied after focal cerebral ischemia in mice reduces activated microglia in the peri-infarct cortex as well as infiltrating mononuclear cells and neutrophils in both peri-infarct cortex and striatum (Peruzzotti-Jametti et al., 2013). Multi-session cathodal tDCS applied for ten consecutive days after stroke in the rat accelerates recovery of function and a shift in microglia polarization (Braun et al., 2016). However, all of these studies were conducted in young rodents in contrast to the older human stroke population. Moreover, chronic neuroinflammatory processes may go on for even 6–12 months or longer after a stroke (Walberer et al., 2014).
Conclusions and recommendations: Current data suggest that both anti-inflammatory and pro-inflammatory effects of TES depend on pre-existing inflammation and TES current density. TES seems to not only affect activation levels of brain-resident and invading immune cells, but also alter their specific phenotype and polarization. However, current research in animals used between 4 and 200 kC/m2 charge densities, which is about 10–500 times higher than levels of tDCS given in humans so far (Liebetanz et al., 2009). For currently applied protocols, there are no hints for neuroinflammations in human studies. So far, tDCS studies did not intend to address long-term chronic neuroinflammatory processes, but rather focused on transient neuroinflammatory response, such as occurring in the sub-acute phase after stroke, or were geared toward promoting neuroplastic processes or cortical excitability changes. More research is needed in this field and the interpretation in term of “changes in neuroinflammation” should be treated with caution.
4. Modeling (heating, induced voltages)
Computational models of current flow relate tDCS surface dose with subject-specific brain current density (Peterchev et al., 2012; Ruffini et al., 2014; Truong et al., 2013). The precision of the prediction depends on the accuracy of the model (not simply the com plexity; (Bikson and Datta, 2012). For a given electrode montage, increasing the current results in a proportional increase in the EF throughout the head – such that, for any given montage, 2 mA will produce an EF in each brain region double that with 1 mA. The local tissue current density is equal to the EF multiplied by the tissue’s conductivity, and thus follows the above dose-response rule for the EF. Because current density is predicted to be much higher in the skin than in the brain, and assuming equal sensitivity to injury of skin and brain, lack of skin injury may indirectly support the claim that the brain current flow is safe (Bikson et al., 2016; Faria et al., 2011; Saturnino et al., 2015).
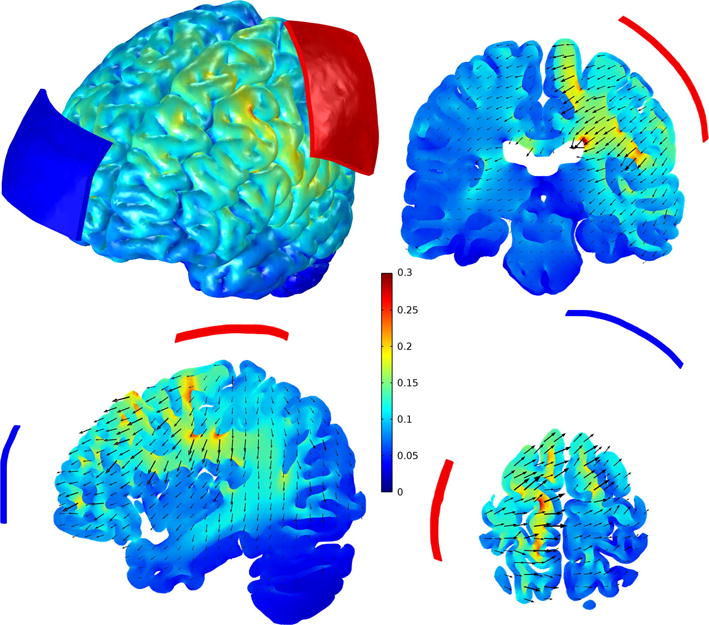
All models predict that the EF in the cortex is strongly affected by the complex arrangement of its folds and by the electrode montage (e.g., Datta et al., 2009a; Miranda et al., 2013; Opitz et al., 2015; Parazzini et al., 2011; Sadleir et al., 2010; Salvador et al., 2010; Wagner et al., 2014a). The EF generally decreases with distance from the electrodes but is non-uniform, with hotspots on the crowns of the gyri that lie between and close to the electrodes, and at the bottom of sulci under the electrodes (Fig. 1). Computational approaches are available to calculate maximal current densities in any area in the brain with a defined stimulation parameter space (Bortoletto et al., 2016; Lee et al., 2016; Seibt et al., 2015; Wagner et al., 2016).
Magnitude of the electric field in the cortex, in V/m. The maximum value of the electric field in the cortex was 0.34 V/m. The 7 × 5 cm2 electrodes were placed over the left hand knob and above the contralateral eyebrow, and the current was set to 1 mA. The three slices pass through the center of the hand knob.
Changes in individual EF distribution can also be calculated in the presence of skull defects or skull plates (Datta et al., 2010), in stroke patients with large defects filled by CSF (Datta et al., 2011) and in children with thinner skulls (Gillick et al., 2014; Kessler et al., 2013; Parazzini et al., 2015). For typical bipolar montages, and in the absence of skull defects or brain lesions, the values predicted for the maximum EF strength in the cortex of realistic head models often fall between 0.2 and 0.5 V/m using 1 mA (e.g., Datta et al., 2009a; Metwally et al., 2015; Miranda et al., 2013; Parazzini et al., 2017; Rampersad et al., 2014; Saturnino et al., 2015; Shahid et al., 2013). The maximal value so far reported by some investigators in a normal brain is 1.6 V/m and can be attributed to the conductivity values used in this particular model (Parazzini et al., 2011). Anatomical variations can have a substantial impact on field strength (Datta et al., 2012; Kessler et al., 2013; Laakso et al., 2015; Truong et al., 2013) and may lead to variations by a factor of 2 or more for a fixed stimulation intensity. Predicted EF strengths of about 0.4 V/m in the cortex are in good agreement with data obtained in epilepsy patients with EF strengths of 0.6–1.6 V/m per 1 mA (Dymond et al., 1975), and ≤0.5 V/m per 1 mA (Opitz et al., 2016). These EFs may be sufficient to modulate neuronal network activity in hippocampal slices (~0.3 V/m, Francis et al., 2003), or to induce entrainment at low frequencies in neocortical slices (~0.7 V/m, Anastassiou et al., 2011). They are slightly lower than the endogenous EFs measured in the ferret’s neocortex (~3 V/m, Frohlich and McCormick, 2010).
The EF strength and its spatial distribution in tACS are expected to be similar to that observed with tDCS. It remains unclear whether the high electric permittivity of brain tissues can significantly affect the strength of the EF in the brain and shift the phase of the sinusoidal waves, in particular with higher frequencies (Logothetis et al., 2007; Opitz et al., 2016; Wagner et al., 2014b). Montages with (multiple) small electrodes do not affect the maximal V/m range with respect to safety considerations (Dmochowski et al., 2011, 2013; Edwards et al., 2013; Ruffini et al., 2014; Sadleir et al., 2012). Because electric current is conducted about 10 times better tangentially along a fiber than perpendicular to it, computational models can take fiber orientation into account by calculating on the basis of diffusion tensor image data in the MRI (e.g., free shareware www.simnibs.de) (Metwally et al., 2012; Opitz et al., 2015; Shahid et al., 2013, 2014).
Heating of the brain during tDCS is considered to be insignificant. For a current of 1 mA, and assuming an EF strength of 0.5 V/m and a conductivity of 0.4 S/m, the power dissipated in the cortex would be about 0.1 mW/kg, which is 5 orders of magnitude less than the metabolic heat production rate in the brain, which is about 11 W/kg (Nelson and Nunneley, 1998). Assuming that the resistance of the extracranial tissue between the two electrodes is about 300 Ω, then the total power dissipated in the whole head would be 0.3 mW. In practice, the resistance between the two electrodes is more likely to be around 10 kΩ due to the contact impedance at the electrode-skin interfaces. In this case, the total power would be 10 mW, dissipated almost entirely in the scalp under the electrode edges. In agreement with these considerations, Datta et al. (2009b) predicts no significant temperature increase (ΔT < 0.003 °C) in the brain or in the scalp for conventional or multichannel tDCS montage for current intensities currently employed. Using multichannel tDCS, several brain regions are targeted in parallel using e.g., arrays of small electrodes on the scalp.
Conclusions and recommendations: Current flow calculation models allow a reasonable estimation of the electric field and current density, including in deep brain areas. Models also allow the design of new montages including electrode arrays. Therefore, EF modeling for targeting predefined areas for stimulation can be helpful. The main potential strength of modeling lies in subject-specific current optimization, which may lead to more reproducible results across individuals and increased safety.
5. Electrode design for TES
A bipolar electrode configuration is the minimal requirement and customarily used for tDCS, with one target electrode placed over the site of the desired cortical stimulation and one remote “return” electrode (but see: Bikson et al., 2010). The return electrode may be placed on the scalp (the most frequently used site), concentrically around the target electrode (Laplacian montage) (Bortoletto et al., 2016; Datta et al., 2009a), extracephalically (e.g., Moliadze et al., 2010; Schambra et al., 2011) or distributed over several sites (Faria et al., 2009).
These electrodes are typically made of conducting materials, some using plastic such as conductive (filled) silicone, while others are metal, usually non-polarizable silver/silver chloride (Ag/AgCl) (Faria et al., 2012; Minhas et al., 2010). The size of the electrode contact area (which for tDCS/tACS is defined as the electrolyte/skin interface) ranges between about 1 cm2 and up to about 100 cm2 (Bortoletto et al., 2016; Ho et al., 2016; Kronberg and Bikson, 2012; Nitsche et al., 2007a). Target and return electrode may be differentiated by size and thus current density, but for bipolar montages the total current is equal across electrodes. Neurophysiological studies indicate that smaller electrodes produce more targeted outcomes while larger electrodes decrease the current density below a given stimulation threshold, such that tDCS no longer has a physiological effect (Nitsche et al., 2007a). Imaging and modeling suggest that electrode placement may play a more significant role than size (Faria et al., 2011).
A recent study has compared scalp sensations using the classical bipolar and HD-tDCS montages over the prefrontal cortex using 1 mA for 20 min (Hill et al., 2017). Stronger sensations were reported after 5 min of stimulation with HD-tDCS compared to either bipolar tDCS or sham tDCS, and this is likely due to the higher current densities produced with this montage using smaller electrodes. After 15 min of stimulation, sensations did not differ between the three conditions and participants were not able to guess at a level better than chance, which type of stimulation they had received.
Conclusions and recommendations: A multitude of possible electrode placements, using either bipolar montage or arrays, permit shaping current flow patterns through the head or targeted stimulation of cortical areas. From available data, no specific safety issues apply for different electrode designs used in tDCS studies. There is no evidence for brain injury following conventional tDCS and multichannel-tDCS protocols. The low to moderate scalp sensation ratings documented in these studies indicate a good overall level of stimulation tolerability provided proper electrode design, preparation, and conventional dose guidance are followed (Woods et al., 2016). For extended protocols (higher intensities, longer duration), a rationale should be given, and it would be advantageous to gather safety information systematically for these protocols before extensive human applications (Bikson et al., 2016).
5.1. Electrochemistry of electrodes
The electrode acts as a transducer between the electron currents in the technical system (stimulator) and the ion currents in the biological system (body). Current can be transmitted across the electrode/electrolyte interface by capacitive charging of the Helmholtz double layer or by electrochemical (faradaic) reactions (Cogan, 2008). Even with large electrodes and thus very low charge densities, one cannot inject a DC of 1–2 mA over a period of several minutes by capacitive charging alone. For instance, the Helmholtz double layer of a 6 cm2 electrode has a capacitance of ca. 120 μF (Kronberg and Bikson, 2012). To charge such a capacitance with a constant current of 1 mA for 15 min would require a voltage of up to 7500 V (1 mA * 15 min/120 μF). The Helmholtz double layer reaction is not associated with any transfer of charge carriers across the interface, but results in an increase in the electrode potential (overpotential), which may cause the onset of unwanted electrochemical reactions such as gas formation by hydrolysis. This is of importance in implanted systems such as cochlear or retinal implants, where the net electrochemical reactions at the electrode interface must be kept at an absolute minimum in order to avoid hydrolysis and electrode corrosion (Merrill et al., 2005). For this reason, invasive neural stimulation is usually performed with very short (60–1000 μs, Howell et al., 2015), charge-balanced, biphasic pulses, in which a cathodic pulse that induces the desired neural stimulation is followed by an anodic pulse to reverse the electrochemical reactions. The charge injection capacity is defined as the maximum charge per pulse and electrode area that can be “safely” injected with an electrode without inducing irreversible electrochemical reactions that would cause electrode corrosion and/or tissue damage. It is mainly dependent on the electrode material and can reach values of several mC/cm2 for materials such as iridium oxide or conductive polymers (Cogan, 2008). Since the capacitive charging is limited to about 20 μF/cm2 (Merrill et al., 2005), and since in transcranial stimulation larger current densities are usually required, capacitive charging of the Helmholtz double layer does not play a major role.
Biphasic sinusoidal pulse currents are mostly used for tACS. Due to the large electrode areas in transcranial applications, the applied charge densities are low (<100 μA/cm2), resulting in an injected charge density of less than 1 μC/cm2 per phase (Woods et al., 2016). No irreversible electrochemical products are known to accumulate at the electrode with such low current densities, although the effective phase (“pulse”) duration during low-frequency tACS (e.g., 1 Hz tACS has a 500 mS phase duration) is much longer and increases the possibility of irreversible reactions. Sinusoidal stimulation is thus not used for implants. The electrodes used for tACS are adapted from tDCS and, hence, provide the same compensation for any potential electrochemical changes. tRNS is not considered here in detail but the use of high-rate charge-balanced pulsing would minimize concerns about electro chemical changes (Merrill et al., 2005), and tRNS seems relatively well tolerated by subjects (Ambrus et al., 2010; Curado et al., 2016; Terney et al., 2008).
In the case of tDCS, current across the interface is unidirectional, of course, and neural stimulation paradigms such as the above mentioned charge injection capacity (Cogan, 2008; Merrill et al., 2005) can therefore not be safely transferred directly to this type of stimulation. The use of DC for stimulation does not allow for reversal of electrochemical reactions during stimulation, but effects such as corrosion and hydrolysis at the electrode may not have as severe consequences for the patient as with implantable stimulators. The essential aspect of electrodes used for tDCS (and tACS) is that metal or conductive rubber where electrochemical reactions may occur are not placed directly on the skin; an electrolyte (saline of gel) always separates the two (Minhas et al., 2010). Therefore, in TES, the current is mainly injected by faradaic reactions but the products of these reactions are kept away from the skin. Conductive rubber electrodes are convenient for macro tDCS/tACS as they are flexible and can be inserted into a saline soaked sponge “pocket”. As an alternative, especially when smaller electrodes are used (e.g., for multichannel stimulation), Ag/AgCl electrodes are well suited due to their non-polarizable character, i.e., their low faradaic resistance results in almost no capacitive charging of the double layer (Merrill et al., 2005). This keeps the electrode potential constant, preventing unwanted faradaic reactions such as gas formation. The reaction mainly responsible for charge transmission at the Ag/AgCl electrode is the formation of AgCl by dissolution and oxidation of solid silver at the anode, and the formation of solid silver by decomposition of AgCl along with a reduction of silver ions at the cathode (Merrill et al., 2005; Minhas et al., 2010). The formation of AgCl requires a suffi-cient amount of free chloride ions in the vicinity of the electrode, which is provided by the electrode gel applied between the electrode and the skin. For this reason, electrode gels containing Cl ions are typically used with Ag/AgCl electrodes.
Small Ag/AgCl electrodes (1–3 cm2, 1–2 mA) with electrode gel, typically containing salts, such as sodium chloride or potassium chloride, are being used more frequently for tDCS with no AEs (e.g. Borckardt et al., 2012; Faria et al., 2012; Murray et al., 2015). Twenty minutes of real (n = 13) or sham (n = 11) 2 mA HD-tDCS over the motor cortex using 1 cm2 electrodes (Borckardt et al., 2012) or 3 × 20 min sessions with 1–2 mA using 3 cm2 PiStim electrodes (hybrid Ag/AgCl EEG/tDCS electrodes with a circular contact area, Starstim, Neuroelectrics) (Murray et al., 2015) resulted in no AEs.
For the sponge electrode design the function of the sponge is to fix the conductive rubber away from the skin and contain the saline. The salinity is important (Dundas et al., 2007), and gel can be substituted for saline. When using a paste electrolyte the sponges may not be necessary but then extreme care must be taken to ensure the conductive rubber does not accidently push through and contact the skin. For HD designs, a holder fixes the distance between the Ag/AgCl electrode and the skin, and also holds the gel. The composition of the electrolyte (saline, gel, or paste) is important as it influence the uniformities of current flow through the skin as well as acting as a chemical (diffusion) buffer between changes at the surface of the metal/rubber and skin (Dundas et al., 2007; Kronberg and Bikson, 2012; Minhas et al., 2010). For both sponge-based Ag/AgCl electrodes the materials and shapes of electrode assembly are thus critical for burden (Minhas et al., 2010). Equally important is adherence to established protocols for electrode preparation and application (Woods et al., 2016).
Recommendations: Use either sponge-like electrodes soaked in saline solution that contain an electrode pad made of conductive rubber (filled silicone), or Ag/AgCl electrodes with appropriate cream. Tap water is not recommended, and care should be taken, even when using saline solution in longer lasting experiments as increased contact resistance may also arise from drying of the sponges (Woods et al., 2016). In such cases an electrode gel or cream is a possible alternative. Abrading the skin (scalp) before electrode placement is not recommended (Loo et al., 2011).
Read more at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5985830/